BACKGROUND: Angiogenesis is increasingly known to play a role in pathogenesis of hematologic malignancies, including myeloid neoplasms. Regorafenib is a multikinase inhibitor that targets angiogenic, stromal and oncogenic kinases including VEGF- 1, 2, 3, TIE-2, PDGFR-β,c-KIT, rRET, RAF-1, FGFR-1, BRAF and p38 MAP kinase. As a result of regorafenib's broad inhibition of kinases and its effects on angiogenesis, this drug has the potential to overcome the limitations of more selective kinase inhibitors and may be associated with efficacy in various myeloid neoplasms, including acute myeloid leukemia (AML), myelodysplastic syndrome (MDS), and myeloproliferative neoplasms (MPN).
METHODS: We conducted a single-center, open-label, dose-escalation and expansion phase I trial in patients with relapsed/refractory AML, MPN, or MDS to assess the safety, tolerability, and preliminary efficacy of regorafenib and identify the recommended phase 2 dose (RP2D). A 3+3 dose escalation design was used with 2 planned dose levels (120 mg or 160 mg daily in 28-day cycles), as well as 1 de-escalation level (80 mg daily). An additional 10 patients were treated on an expansion cohort.
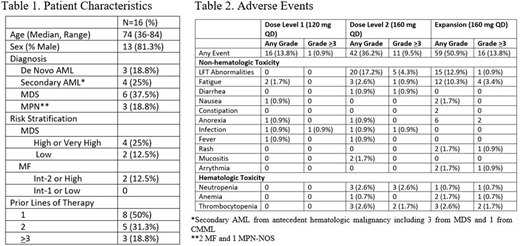
RESULTS: Six patients were enrolled during the dose escalation phase (3 at 120 mg daily followed by 3 at 160 mg daily), with no DLTs, as defined by a Grade >3 toxicity occurring within the first 28 days after initiation of treatment, and unrelated to the myeloid neoplasm. Therefore, the RP2D of regorafenib was identified as 160 mg daily. Ten additional patients were enrolled on this dose during the expansion phase. The median age was 74 (range 36-84), and 13 (81%) patients were male. Diagnoses included 7 AML, 6 MDS, and 3 MPN patients (Table 1). Dose modifications and delays occurred in 5 and 4 patients, respectively. The median duration of treatment was 56 days or 2.5 cycles (range: 5-410 days, 1-15 cycles).
Of the 16 patients, the best overall disease response as measured by IWG criteria included partial remission (in 1 patient with AML), stable disease in 12 (2 de novo AML, 2 secondary AML, 3 MPN, and 5 MDS) patients, and progressive disease in 3 (1 MDS and 2 secondary AML) patients. An initial improvement in absolute neutrophil count and/or hemoglobin was observed in the 6 MDS patients, although this did not meet criteria for hematologic improvement by IWG criteria. All patients have discontinued off treatment. The most frequent Grade 3-4 adverse effects (AEs) included liver function test abnormalities (5.1%), fatigue (4.3%), thrombocytopenia (3.4%), and neutropenia (3.4%) (Table 2). The majority of AEs were grades 1-2.
CONCLUSIONS: Regorafenib demonstrates an acceptable safety profile in relapsed/refractory myeloid malignancy patients, a population where few treatment options exist. The majority of patients achieved stable disease. Modest improvements in cell counts were observed in MDS patients, and we are performing correlative studies to clarify regorafenib's mechanism of action and identify populations which may benefit from treatment.
Amrein:AstraZeneca: Consultancy, Research Funding; Takeda: Research Funding; Amgen: Research Funding. Brunner:Novartis: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; AstraZeneca: Research Funding; Forty-Seven Inc: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Fathi:Kura Oncology: Consultancy; Trillium: Consultancy; Boston Biomedical: Consultancy; Pfizer: Consultancy; Daiichi Sankyo: Consultancy; Jazz: Consultancy; Agios: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; PTC Therapeutics: Consultancy; Amphivena: Consultancy; Astellas: Consultancy; Amgen: Consultancy; Seattle Genetics: Consultancy, Research Funding; Abbvie: Consultancy; Blueprint: Consultancy; Kite: Consultancy; Trovagene: Consultancy; Forty Seven: Consultancy; Newlink Genetics: Consultancy; Novartis: Consultancy; BMS/Celgene: Consultancy, Research Funding. Narayan:Sanofi-Genzyme: Other: Current Spouse employment ; Takeda: Other: Prior Spouse employment within 24 months; Genentech: Other: Prior Spouse employment within 24 months and prior spouse equity divested within past 24 months. Neuberg:Pharmacyclics: Research Funding; Madrigak Pharmaceuticals: Current equity holder in publicly-traded company; Celgene: Research Funding. Chen:Takeda: Consultancy; Magenta: Consultancy; Equillium: Other: Data and Safety Monitoring Board Member; AbbVie: Other: Data and Safety Monitoring Board Member; Incyte Corporation: Consultancy; Actinium: Other: Data and Safety Monitoring Board Member; Kiadis: Consultancy. Hobbs:Merck: Research Funding; Constellation: Honoraria, Research Funding; Incyte: Research Funding; Jazz: Honoraria; Novartis: Honoraria; Celgene/BMS: Honoraria; Bayer: Research Funding.
Regorafenib was used to assess safety and preliminary efficacy in advanced myeloid malignancies
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal